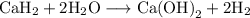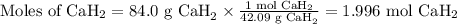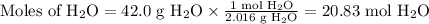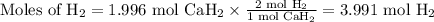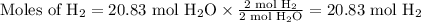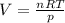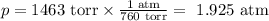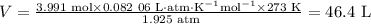Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:00, aubreymoore9441
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1


Chemistry, 23.06.2019 02:20, theactualslash
In a chemical reaction, the final amount of the products is determined by the a. universal gas law b. law of definite proportions c. air pressure d. temperature e. none of the above me
Answers: 2
You know the right answer?
Beginning with 84.0 g of cah2 and 42.0 g of h2o, what volume of h2 will be produced at 273 k and a p...
Questions in other subjects:

Social Studies, 21.12.2020 05:30

Mathematics, 21.12.2020 05:30

Mathematics, 21.12.2020 05:30





English, 21.12.2020 05:30

English, 21.12.2020 05:30

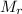 : 42.09 18.02 2.016
: 42.09 18.02 2.016