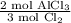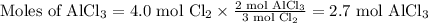Chemistry, 05.07.2019 02:30 jadetaull19
According to the balanced equation below, which of the following is equivalent to how many moles of alcl3 can be made from 4.0 moles of cl2? 2al + 3cl2 > 2alcl3a) 2.7 molesb) 4.0 molesc) 8.0 molesd) 24 moles

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 17:10, sophiaa23
)benzene and toluene form nearly ideal solutions. consider an equimolar solution of benzene and toluene. at 20 °c the vapour pressures of pure benzene and toluene are 9.9 kpa and 2.9 kpa, respectively. the solution is boiled by reducing the external pressure below the vapour pressure. calculate (i) the pressure when boiling begins, (ii) the composition of each component in the vapour, and (iii) the vapour pressure when only a few drops of liquid remain. assume that the rate of vaporization is low enough for the temperature to remain constant at 20 °c.
Answers: 1


Chemistry, 23.06.2019 08:00, mackaylabarnes22
Ineed this awnser fast select the correct answer. this chemical equation represents the burning of methane, but the equation is incomplete. what is the missing coefficient in both the reactants and the products? ch4 + → co2 + a. 0 b. 1c. 2d. 3 e. 4
Answers: 3
You know the right answer?
According to the balanced equation below, which of the following is equivalent to how many moles of...
Questions in other subjects:





World Languages, 25.02.2020 23:54





 or
or