
2suppose that a new element (e) were discovered that existed as three natural isotopes: 25% of atoms had a mass of 278, 38% had a mass of 281, and the remained had a mass of 285. what would be listed as the atomic mass of this element? explain your logic and show all steps.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, joelpimentel
This flow chart shows the amount of energy that is emitted by each type of light. ultraviolet > blue light > yellow light > red light (maximum energy) (minimum energy) in an experiment, shining which type of light on a strip of metal would be least likely to produce the photoelectric effect? ultraviolet light dim blue light bright red light bright yellow light
Answers: 2

Chemistry, 22.06.2019 09:00, triddi666
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 19:00, cindyroxana229
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3

Chemistry, 22.06.2019 19:30, periwinkleaqua72
What is the mass of oxygen gas is consumed in a reaction that produces 4.60mol so2
Answers: 3
You know the right answer?
2suppose that a new element (e) were discovered that existed as three natural isotopes: 25% of atom...
Questions in other subjects:

Business, 16.01.2020 22:31

Physics, 16.01.2020 22:31

Physics, 16.01.2020 22:31



History, 16.01.2020 22:31


Social Studies, 16.01.2020 22:31


Mathematics, 16.01.2020 22:31

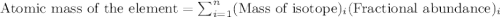 .....(1)
.....(1)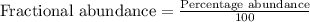
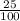 =0.25
=0.25![\text{Atomic mass of the element}=\left[(278\times 0.25)+(281\times 0.38)+(285\times 0.37) \right ]](/tpl/images/0052/4546/3e73a.png)


