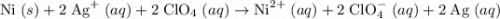Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, microwave13016
Agroup of students is studying convection current. they fill two identical balloons with the same amount of helium. one balloon is placed in a freezer and the other is in an area with warm air. after 10 minutes, the balloon are released from a height of 1 meter. which of the following to the students most likely observe? a) the warm balloon expands and rises. the cold balloon shrinks and sinks b) the balloon both rise. the cold balloon is larger than the warm balloon c) the cold balloon expands and rises. the warm balloon shrinks and sinks d) the balloon rise at the same rate. both balloons are the same size
Answers: 1

Chemistry, 22.06.2019 22:30, robertss403
How many moles of kci are produced from 2.50 moles k
Answers: 1

Chemistry, 22.06.2019 23:00, DESI111609
What is the average rate of the reaction between 10 and 20 s?
Answers: 1

You know the right answer?
Determine the oxidizing agent in the following reaction. ni(s) + 2 agclo4(aq) → ni(clo4)2(aq) + 2 ag...
Questions in other subjects:


Mathematics, 19.01.2020 06:31

Mathematics, 19.01.2020 06:31

Mathematics, 19.01.2020 06:31

Biology, 19.01.2020 06:31

Mathematics, 19.01.2020 06:31

Chemistry, 19.01.2020 06:31


English, 19.01.2020 06:31

English, 19.01.2020 06:31

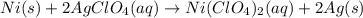
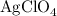 serves as the oxidizing agent in this reaction.
serves as the oxidizing agent in this reaction.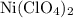 in the products exists as ionic salt dissolved in an aqueous solution. Rewriting the chemical equation as an ionic one might help eliminate spectator ions and make changes in oxidation states more apparent.
in the products exists as ionic salt dissolved in an aqueous solution. Rewriting the chemical equation as an ionic one might help eliminate spectator ions and make changes in oxidation states more apparent.