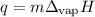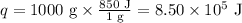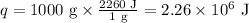Chemistry, 05.07.2019 10:30 dontcareanyonemo
Ethyl alcohol has a latent heat of vaporization of 850 j/g. water has a latent heat of vaporization of 2260 j/g. container a contains 1.0 kg of ethyl alcohol. container b contains 1.0 kg of water. both liquids are brought to a boil. if the same amount of heat is continuously added to each container, which liquid will boil away first? a ) water b ) ethyl alcohol c ) both will boil away at the same time d )boiling and condensation will happen at the same rate so neither liquid will boil away

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 12:30, kylieweeks052704
Achemist determined by measurements that 0.0300 most of beryllium oxide participate in a chemical reaction calculate the mass of berlylium oxide that participates
Answers: 3


Chemistry, 23.06.2019 08:30, zhjzjzzj8225
Explain how to convert from one unit to another in the metric system.
Answers: 3
You know the right answer?
Ethyl alcohol has a latent heat of vaporization of 850 j/g. water has a latent heat of vaporization...
Questions in other subjects:



Mathematics, 29.03.2021 21:40

Physics, 29.03.2021 21:40