

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, ciel8809
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1


Chemistry, 22.06.2019 21:30, djdjdjdbdbjx
What is another way to determine mass times acceleration?
Answers: 1
You know the right answer?
In standardizing the solution of aqueous sodium hydroxide, a chemist overshoots the end point and ad...
Questions in other subjects:



Mathematics, 16.10.2020 20:01



Mathematics, 16.10.2020 20:01

Mathematics, 16.10.2020 20:01


History, 16.10.2020 20:01

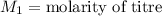 ,
, 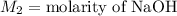
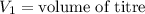 ,
, 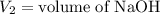
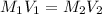
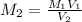 ....(1)
....(1)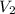 .
.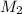 in equation (1), will get lowered , which means that the concentration of NaOH was lower than that of the actual value. Hence underestimated concentration of NaOH.
in equation (1), will get lowered , which means that the concentration of NaOH was lower than that of the actual value. Hence underestimated concentration of NaOH. 

