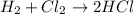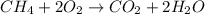Chemistry, 05.07.2019 12:30 izzysmith6836
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2 + 2h2o equation a represents a reaction, and equation b represents reaction. choices for both blanks combustion decombustion synthesis double displacement single displacement choose one for each blank there are some notes and examples in the images my opinions are 1. 2blank--combustion tell me if im correct 16pionts

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, lazavionadams81
Identify which properties could correspond to solids, plasmas, or both. maintain a unique shape. collide infrequently with other particles. have very high velocities. conduct electricity. protons. have a low temperature. has long-range order.
Answers: 1

Chemistry, 22.06.2019 01:30, arodavoarodavo
The table lists pressure and volume values for a particular gas. which is the best estimate for the value of v at p = 7.0 × 103 pascals?
Answers: 3

Chemistry, 22.06.2019 12:00, sophiaa23
Which of the following is an example of physical change not a chemical change? a) a log gives off heat and light as it burns. b) a tree stores energy from the sun in its fruit. c) a penny lost in the grass slowly changes color. d) a water pipe freezes and cracks on a cold night.
Answers: 2
You know the right answer?
Identify the type of reaction represented by each equation. a: h2 + cl2 → 2hcl b: ch4 + 2o2 → co2...
Questions in other subjects:


Mathematics, 11.02.2021 20:30


English, 11.02.2021 20:30



Chemistry, 11.02.2021 20:30