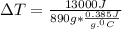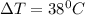Chemistry, 05.07.2019 18:00 calebmoore925
A1.1 kg block of iron at 38 ∘c is rapidly heated by a torch such that 13 kj is transferred to it. what temperature would the block of iron reach assuming the complete transfer of heat and no loss to the surroundings? if the same amount of heat was quickly transferred to a 890 g pellet of copper at 38 ∘c, what temperature would it reach before losing heat to the surroundings? cs, fe(s)= 0.450 j/g*c cs, cu(s)= 0.385 j/g*c

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, ashlpiriz123
Find the number of grams of hcl needed to react completely with .50 moles of magnesium.
Answers: 1

Chemistry, 22.06.2019 11:00, coco8560
Freezing and boiling are endothermic processes. this means that these processes absorb energy from their surroundings in order to occur. use this information and the data you collected in the phase change gizmo to describe what happens to the temperature of water when you boil it, then explain why this result occurs.
Answers: 1

Chemistry, 22.06.2019 17:10, mikeeway33
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

You know the right answer?
A1.1 kg block of iron at 38 ∘c is rapidly heated by a torch such that 13 kj is transferred to it. wh...
Questions in other subjects:

Mathematics, 30.06.2019 15:50

Social Studies, 30.06.2019 15:50




Geography, 30.06.2019 15:50



Social Studies, 30.06.2019 15:50

Mathematics, 30.06.2019 15:50

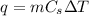
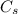 is specific heat and
is specific heat and  is the change in temperature.
is the change in temperature.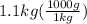 = 1100 g
= 1100 g = 13000 J
= 13000 J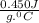
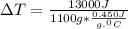
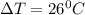
 .
.