
Chemistry, 06.07.2019 06:00 preservations
If 6.500 g of c6h6 is burned and the heat produced from the burning is added to 5691 g of water at 21 °c, what is the final temperature of the water?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 23.06.2019 01:00, daniel1480
Which fossil fuel is mainly used for heating and cooking? a. electricity b. coal c. petroleum d. natural gas
Answers: 2


Chemistry, 23.06.2019 22:30, ashlpiriz123
What is the amount of energy needed to change the temperature of 0.20 kilogram of lead from 20 degree celsius to 30 degree celsius.
Answers: 1
You know the right answer?
If 6.500 g of c6h6 is burned and the heat produced from the burning is added to 5691 g of water at 2...
Questions in other subjects:

Mathematics, 03.03.2021 18:20




Mathematics, 03.03.2021 18:20

English, 03.03.2021 18:20




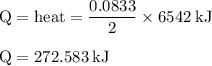
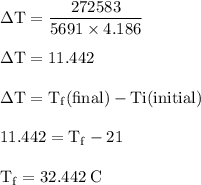 -
- 

