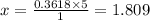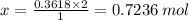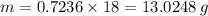Chemistry, 06.07.2019 06:30 jscout2468
You are given the balanced chemical equation: c4h4 + 5o2 4co2 + 2h2o. if 0.3618 moles of c4h4 are allowed to react with 1.818 moles of o2, and this is the only reaction which occurs, what is the maximum mass of water that could be produced?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 13:10, bartonamber4042
Select the correct answer a modure consists of glucose and water. what is the percent composition of glucose in the mixture if it contains 1.3 moles of glucose (cho total mass of the mature is 276 grams? ) and the a 1775
Answers: 1

Chemistry, 22.06.2019 16:50, struckedblazing
Answer asap need it by wednesday morning calculate the ph of 0.02m hcl best answer will be brainliest
Answers: 1
You know the right answer?
You are given the balanced chemical equation: c4h4 + 5o2 4co2 + 2h2o. if 0.3618 moles of c4h4 are a...
Questions in other subjects:

English, 12.12.2020 15:50

Biology, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50



German, 12.12.2020 15:50

Mathematics, 12.12.2020 15:50

History, 12.12.2020 15:50