

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, Makoshark6887
According to coulomb's law, how would the electrical force between particles change if the product of their electrical charge increased?
Answers: 1

Chemistry, 22.06.2019 16:00, sassy11111515
The chemical equation below shows the reaction of sodium (na) and chlorine (cl) to form sodium chloride (nacl). 2na + cl2 → 2nacl in this equation, which of the following is a reactant? i. sodium ii. chlorine iii. sodium chloride
Answers: 1

Chemistry, 23.06.2019 09:20, goldwinner300
Asolution of naoh has a concentration of 25.00% by mass. what mass of naoh is present in 0.250 g of this solution? use the periodic table in the toolbar if needed. 0.0625 g what mass of naoh must be added to the solution to increase the concentration to 30.00% by mass? g
Answers: 2

Chemistry, 23.06.2019 12:40, valleriieZ7002
Metric temperature is measured in celsius and fahrenheit. true or false
Answers: 2
You know the right answer?
If 5 moles of p4 reacted with 22 moles cl2 according to the above reaction, determine:
a. how...
a. how...
Questions in other subjects:


Mathematics, 26.10.2020 22:20

Mathematics, 26.10.2020 22:20

Mathematics, 26.10.2020 22:20

Mathematics, 26.10.2020 22:20

Mathematics, 26.10.2020 22:20


Mathematics, 26.10.2020 22:20

Mathematics, 26.10.2020 22:20


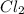 gives 4 mole of
gives 4 mole of  , then 22 moles of
, then 22 moles of  moles of
moles of 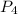 , then 22 moles of
, then 22 moles of 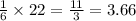 moles of
moles of  moles.
moles.


