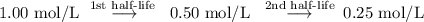Chemistry, 06.07.2019 15:30 esanchez2002fcb
Afirst-order reaction has a half-life of 16.7 s . how long does it take for the concentration of the reactant in the reaction to fall to one-fourth of its initial value?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, natalie857123
For each of the following types of reactions, write a general reaction formula in the symbolic form—for example, a + b → ab. single-displacement double-displacement synthesis decomposition
Answers: 1

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
Afirst-order reaction has a half-life of 16.7 s . how long does it take for the concentration of the...
Questions in other subjects:

History, 10.03.2020 21:34








Geography, 10.03.2020 21:35

History, 10.03.2020 21:35