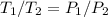Chemistry, 06.07.2019 17:00 miguel454545
Explain how you can use boyle's law to determine the new volume of gas when it's pressure is increased from 170 to 5: 40 the original volume of gas is one leader assume the temperature and number of particles are consistent what is the new volume

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:00, Dkhaurithompson
Zoe is investigating the composition of substance a, an unknown substance. using chemical processes, she analyzes substance a and determines it is composed of sodium, oxygen, and hydrogen atoms in a ratio of 1 : 1 : 1. what is substance a? a. a compound b. an element c. a heterogeneous mixture d. a homogeneous mixture
Answers: 1


Chemistry, 22.06.2019 10:30, zayam1626
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2

Chemistry, 22.06.2019 11:50, tajanaewilliams77
If oil spills continue, all of the following should be expected except (2 points) death of aquatic life. polluted groundwater. decreased soil productivity. increased global temperatures.
Answers: 3
You know the right answer?
Explain how you can use boyle's law to determine the new volume of gas when it's pressure is increas...
Questions in other subjects:

Mathematics, 20.10.2020 05:01


History, 20.10.2020 05:01

Mathematics, 20.10.2020 05:01

Mathematics, 20.10.2020 05:01



History, 20.10.2020 05:01

Mathematics, 20.10.2020 05:01

 Initial Pressure
Initial Pressure  Final Pressure
Final Pressure