
What does the pauli exclusion principle state?
a) electrons will be added to an atom at the lowest possible level or sublevel.
b) an orbital can contain two electrons only if all other orbitals at that sublevel are empty.
c) an orbital can contain two electrons only if all other orbitals at that sublevel contain at least one electron.
d) two electrons occupy the same orbital only if they have opposite spins.
e) an orbital can contain two electrons only if all other orbitals at that sublevel are empty.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:20, sarinaneedshelp01
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1

Chemistry, 22.06.2019 02:40, gabrielolivas59
The difference between the atomic number of an element and the element’s atomic mass is the number of ions.
Answers: 3

Chemistry, 22.06.2019 14:00, JJlover1892
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 23.06.2019 01:00, davelopez979
An unsaturated hydrocarbon is a hydrogen-carbon compound with a. a network solid structure b. single bonds c. single bonds in a branched-chain structure d. double or triple bonds
Answers: 1
You know the right answer?
What does the pauli exclusion principle state?
a) electrons will be added to an atom a...
a) electrons will be added to an atom a...
Questions in other subjects:

Geography, 21.01.2020 13:31



Mathematics, 21.01.2020 13:31

Social Studies, 21.01.2020 13:31





English, 21.01.2020 13:31

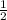 or
or  .
.


