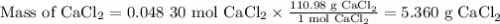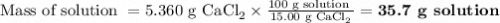Chemistry, 07.07.2019 03:00 staz13wiggins
If you add water to a 15.00 ml solution of 3.22 m cacl2 (mm=110.98 g/mol) in order to create a solution that is 15.00% cacl2 by mass, what is the final mass of the new solution. the density of water is exactly 1.00 g/ml. assume that the density of the cacl2 solution is also exactly 1.00 g/ml.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:30, Aidanjsauer
Check the correct box to describe the periodic trends in electronegativity. electronegativity across a period: decreases. increases. electronegativity down a group: decreases. increases.
Answers: 2

Chemistry, 21.06.2019 22:50, ajaydonlee
Select the correct answer how does the heat content of the reaction change in the process of photosynthesis when a glucose molecule is formed? ca the value of is negative the value of qis positive the value of a remains constant the value of a decreases the value of equals zero e
Answers: 2

Chemistry, 22.06.2019 04:30, jocelynmarquillo1
Acamcorder has a power rating of 17 watts. if the output voltage from its battery is 7 volts, what current does it use?units:
Answers: 1

You know the right answer?
If you add water to a 15.00 ml solution of 3.22 m cacl2 (mm=110.98 g/mol) in order to create a solut...
Questions in other subjects:

Biology, 20.11.2019 13:31


Chemistry, 20.11.2019 13:31


Social Studies, 20.11.2019 13:31

History, 20.11.2019 13:31

Mathematics, 20.11.2019 13:31

Mathematics, 20.11.2019 13:31

Spanish, 20.11.2019 13:31

Mathematics, 20.11.2019 13:31