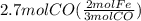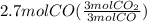Read the chemical equation. fe2o3 + co → fe + co2 if 1.8 moles of fe2o3 react with 2.7 moles of co, how many moles of each product are formed? 5.4 moles fe and 1.8 moles co2 2.7 moles fe and 0.9 moles co2 3.6 moles fe and 5.4 moles co2 1.8 moles fe and 2.7 moles co2

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:30, audrey1256
Which change in temperature is the smallest? a change of 1 thomson degree a change of 1 kelvin degree a change of 1 fahrenheit degree a change of 1 celsius degree
Answers: 1

Chemistry, 22.06.2019 11:30, charles8527
If blood contains 150g of hemoglobin per liter of blood, how much hemoglobin would be contained in 10 ml of blood
Answers: 2

Chemistry, 22.06.2019 11:50, hadwell34
Which of the following statements about hybrid orbitals is or are true? choose all that apply. choose all that apply. under sp2 hybridization, the large lobes point to the vertices of an equilateral triangle. after an atom undergoes sp hybridization there is one unhybridized p orbital on the atom. the angle between the large lobes of sp3 hybrids is 109.5∘
Answers: 2
You know the right answer?
Read the chemical equation. fe2o3 + co → fe + co2 if 1.8 moles of fe2o3 react with 2.7 moles of co,...
Questions in other subjects:





Mathematics, 05.09.2020 04:01

Mathematics, 05.09.2020 04:01

Mathematics, 05.09.2020 05:01



 .
.