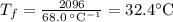Chemistry, 07.07.2019 04:30 IsabellaGracie
What would be the final temperature of the system if 21.2 g of lead at 113 ◦c is dropped into 14.8 g of water at 28.9 ◦c in an insulated container? the specific heat of lead is 0.128 j/g◦c.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, ciarakelly636owuiup
Asample of the male sex hormone testosterone, c19h28o2, contains 3.88×10^21 atoms of hydrogen.(a) how many atoms of carbon does it contain? (b) how many molecules of testosterone does it contain? (c) how many moles of testosterone does it contain? (d) what is the mass of this sample in grams?
Answers: 1



Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2
You know the right answer?
What would be the final temperature of the system if 21.2 g of lead at 113 ◦c is dropped into 14.8 g...
Questions in other subjects:

Mathematics, 20.10.2019 12:50

Spanish, 20.10.2019 12:50

Mathematics, 20.10.2019 12:50


Mathematics, 20.10.2019 12:50


Chemistry, 20.10.2019 12:50

History, 20.10.2019 12:50


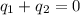
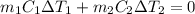
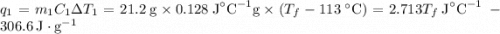
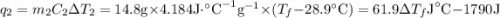
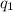 and
and 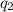 and combinibg like terms, we get
and combinibg like terms, we get![168.0\text{T}_{f}\: ^{\circ}\text{C} ^{-1}\:- 2096 = 0]\\](/tpl/images/0060/4306/42ee8.png)