
Chemistry, 07.07.2019 04:30 Tyrant4life
Type the correct answer in the box. express your answer to three significant figures. this balanced equation shows the reaction of sodium hydroxide and sulfuric acid: 2naoh + h2so4 → na2so4 + 2h2o. in a laboratory experiment, a student mixes 355 grams of sulfuric acid with an excess of sodium hydroxide. what is the theoretical mass of sodium sulfate produced? refer to the periodic table and the polyatomic ion resource. the theoretical mass of sodium sulfate is grams.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:50, adam1299
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments, solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1


Chemistry, 22.06.2019 18:30, chinadoll24
Asample of hydrated tin (ii) chloride (sncl2) has a mass of 4.90 g. when it is dehydrated, it has a mass of 4.10 g. which is the correct chemical formula for the hydrate? sncl2•2h2o sncl2•4h2o sncl2•6h2o
Answers: 2

Chemistry, 22.06.2019 19:30, xxaurorabluexx
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2
You know the right answer?
Type the correct answer in the box. express your answer to three significant figures. this balanced...
Questions in other subjects:


Spanish, 11.10.2019 10:30


Mathematics, 11.10.2019 10:30

Physics, 11.10.2019 10:30

Chemistry, 11.10.2019 10:30




Mathematics, 11.10.2019 10:30

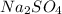 ) is 514.118 grams.
) is 514.118 grams.
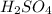 is a limiting reagent as the quantity of the product will depend on it.
is a limiting reagent as the quantity of the product will depend on it.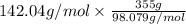 of
of 

