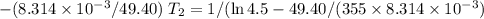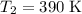Answers: 2


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, laurachealsy923
In an energy pyramid, which level has the most available energy?
Answers: 1

Chemistry, 22.06.2019 02:10, apowers6361
26. of of (aq) by (aq) is . if 50.00 ml of 1.05 m is to 25.00 ml of 1.86 m ,at be? ( no is toina of aof) , h.. (p. ). . .
Answers: 3

Chemistry, 22.06.2019 10:00, aschool2000
Water's surface tension and heat storage capacity are accounted for by its a) orbitals b) weight c) hydrogen bonds d) mass e) size
Answers: 2
You know the right answer?
Acertain reaction has an activation energy of 49.40 kj/mol. at what kelvin temperature will the reac...
Questions in other subjects:

Mathematics, 10.05.2021 19:40


Mathematics, 10.05.2021 19:40

Mathematics, 10.05.2021 19:40


Mathematics, 10.05.2021 19:40

English, 10.05.2021 19:40


Mathematics, 10.05.2021 19:40

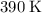
 if the concentration of all reactants in its rate-determining step is held constant. The rate "constant" is dependent on both the temperature and the activation energy of this particular reaction, as seen in the Arrhenius equation:
if the concentration of all reactants in its rate-determining step is held constant. The rate "constant" is dependent on both the temperature and the activation energy of this particular reaction, as seen in the Arrhenius equation: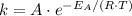
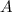 the frequency factor constant unique to this reaction
the frequency factor constant unique to this reaction the base of natural logarithms, and
the base of natural logarithms, and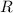 the ideal gas constant.
the ideal gas constant.
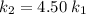 , such that
, such that 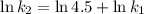
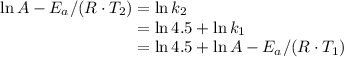
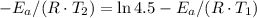
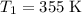 and activation energy
and activation energy 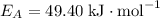 - assumed to be independent of temperature variations,
- assumed to be independent of temperature variations,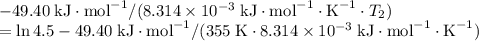
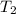 :
: