
Chemistry, 07.07.2019 15:00 gdominguez2005
H+ ions increase in concentration at lower ph values. calculate how many more h+ ions there are in a solution at a ph = 2 than in a solution at a ph = 6. find the concentration of h+ ions at a ph = 2 and at a ph = 6 in table b. then divide the concentration of h+ ions at a ph = 2 by the of h+ ions at a ph = 6. record your answer in table c. what is the concentration of h+ ions at a ph = 2? mol/l what is the concentration of h+ ions at a ph = 6? mol/l how many more h+ ions are there in a solution at a ph = 2 than in a solution at a ph = 6? need asap give brainliest

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 03:20, Richwave17
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 04:00, speris1443
Write the balanced equation for a reaction between aqueous nitric acid (hno3) and solid lithium metal (this is a single replacement reaction)
Answers: 1


Chemistry, 22.06.2019 06:00, mbrisen7420
Compare and contrast physical changes with chemical changes.
Answers: 3
You know the right answer?
H+ ions increase in concentration at lower ph values. calculate how many more h+ ions there are in a...
Questions in other subjects:


Health, 16.09.2019 18:10

Biology, 16.09.2019 18:10

Law, 16.09.2019 18:10






![pH=-log[H⁺]](/tpl/images/0062/1602/22ab6.png)
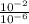 = 10⁴
= 10⁴


