Chemistry, 07.07.2019 18:30 cougar9754
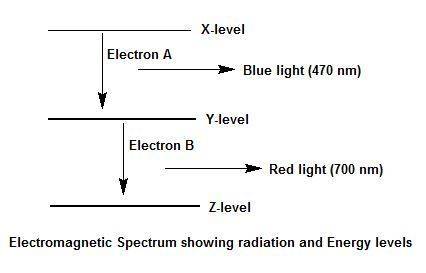
Electron a falls from energy level x to energy level y and releases blue light. electron b falls from energy level y to energy level z and releases red light. which transition, from x to y or from y to z, has a greater energy difference? explain your answer and how you arrived at it. use a diagram of the electromagnetic spectrum.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 13:00, carlinryan
16. why must the number of electrons lost equal the number of electrons gained in every redox reaction? use 3 – 4 sentences in your own words to address this question. 18. what type of radiation is emitted when chromium-51 decays into manganese-51? show the nuclear equation that leads you to this answer. 19. a radioactive nucleus alpha decays to yield a sodium-24 nucleus in 14.8 hours. what was the identity of the original nucleus? show the nuclear equation that leads you to this answer.
Answers: 2

Chemistry, 22.06.2019 13:30, yasiroarafat12
How many moles is 14.5 cm^3 of platinum? the density of platinum is 21.45 g/cm^3.
Answers: 1
You know the right answer?
Electron a falls from energy level x to energy level y and releases blue light. electron b falls fro...
Questions in other subjects:


Mathematics, 07.09.2020 01:01



History, 07.09.2020 01:01

Mathematics, 07.09.2020 01:01


History, 07.09.2020 01:01


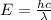
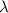 = Wavelength of particle
= Wavelength of particle