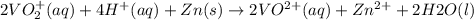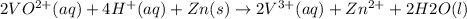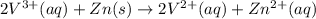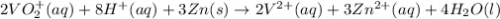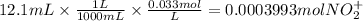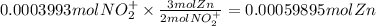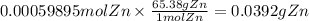Chemistry, 07.07.2019 19:30 tladitidimatso1783
The balanced redox reactions for the sequential reduction of vanadium are given below. reduction from +5 to +4: 2 vo2+(aq) + 4 h +(aq) + zn(s) → 2 vo2+(aq) + zn2+(aq) + 2 h2o(l) reduction from +4 to +3: 2 vo2+(aq) + zn(s) + 4 h +(aq) → 2 v3+(aq) + zn2+(aq) + 2 h2o(l) reduction from +3 to +2: 2 v3+(aq) + zn(s) → 2 v2+(aq) + zn2+(aq) if you had 12.1 ml of a 0.0033 m solution of vo2+(aq), how many grams of zn metal would be required to completely reduce the vanadium?

Answers: 1


Other questions on the subject: Chemistry



Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1

Chemistry, 22.06.2019 21:00, lalaween098
What type of radiation is lead emitting in the following equation? alpha particles beta particles gamma rays
Answers: 3
You know the right answer?
The balanced redox reactions for the sequential reduction of vanadium are given below. reduction fro...
Questions in other subjects:

Biology, 19.05.2021 15:50





Spanish, 19.05.2021 15:50


Mathematics, 19.05.2021 15:50

Biology, 19.05.2021 15:50