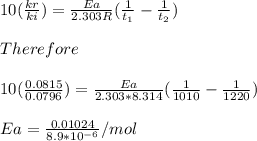Chemistry, 07.07.2019 21:00 madisontrosclair2
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollution generated in automobile engines. the decomposition of nitric oxide (no) to n2 and o2 is second order with a rate constant of 0.0796 m−1⋅s−1 at 737∘c and 0.0815 m−1⋅s−1 at 947∘c. you may want to reference (page) section 14.5 while completing this problem. part a calculate the activation energy for the reaction. express the activation energy in kilojoules per mole to three significant digits.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 13:30, ayoismeisalex
Astudent is trying to create a table that compares hypotheses, theories, and laws. hypothesis theory law do scientific researchers formulate it? yes yes yes does it explain why things happen? yes yes no yes yes yes is it used to make predictions? no yes yes which of the following questions would most likely fill the blank in the table? is it an intelligent guess? is it newly formulated? is it based on observations? has it been proved?
Answers: 1

Chemistry, 23.06.2019 02:30, micahwilkerson9495
Which words or phrases identify layers of groundwater? check all that apply. water table kettle lake saturation zone underground lake sinkhole will give brainiest, answer quickly.
Answers: 1

Chemistry, 23.06.2019 08:20, debramknoxx
At which temperature would a reaction with ah= -220 kj/mol and as=-0.05 kj/(mol-k) be spontaneous?
Answers: 2
You know the right answer?
Understanding the high-temperature behavior of nitrogen oxides is essential for controlling pollutio...
Questions in other subjects:

Spanish, 01.03.2021 22:20


Mathematics, 01.03.2021 22:20



Computers and Technology, 01.03.2021 22:20


Mathematics, 01.03.2021 22:20


Mathematics, 01.03.2021 22:20