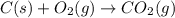The question: a new grill has a mass of 30.0 kg. you put 2.0 kg of charcoal in the grill. you burn all the charcoal and the grill has a mass of 30.0 kg. what is the mass in kg of the gases given off? (assume that the charcoal is pure carbon solid and that it burns completely in oxygen). the mass of carbon burnt = 2kg = 2000g moles of carbon burnt = 2000/12 => moles of co2 produced = 2000/12 => mass of co2 produced = 2000/12*44 = 2 333.33 g = 2.33 kg in the answer, where did the 44 come from?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:30, tiwaribianca475
What volume of a 2.00 m stock solution of naoh is needed to prepare 150. ml of 0.40 m solution?
Answers: 2

Chemistry, 23.06.2019 05:40, girlchamp654
The independent variable in an experiment will be the variable that you o a) change ob) hold constant ng c) observe for changes
Answers: 2

Chemistry, 23.06.2019 09:20, taylorannsalazar
La reaccion entre monoxido de nitrogeno (no) y oxigeno para formardioxido de nitrogeno (no2) es un paso determinante para la formacion del smog, la reaccion es la siguiente: 2no + o2 = 2no2 cual sera el numero de moles de no2 que se formaran por la reaccion completa de 8 moles de oxigeno con suficiente monoxido?
Answers: 1
You know the right answer?
The question: a new grill has a mass of 30.0 kg. you put 2.0 kg of charcoal in the grill. you burn a...
Questions in other subjects:

English, 27.08.2021 14:00


Biology, 27.08.2021 14:00


English, 27.08.2021 14:00


Mathematics, 27.08.2021 14:00

Mathematics, 27.08.2021 14:00

Physics, 27.08.2021 14:00

 .
.