

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:00, kayla32213
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1


Chemistry, 22.06.2019 23:30, znewkirk4741
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3
You know the right answer?
Calculate the number of moles of bromine present in 20.5 ml of br2(l), whose density is 3.12 g/ml....
Questions in other subjects:

English, 18.10.2020 14:01


Advanced Placement (AP), 18.10.2020 14:01

Spanish, 18.10.2020 14:01


Mathematics, 18.10.2020 14:01




Mathematics, 18.10.2020 14:01

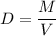
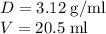
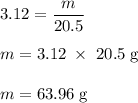
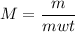
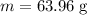
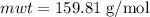
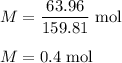
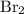 present in 20.5 ml has been 0.4 mol.
present in 20.5 ml has been 0.4 mol.

