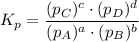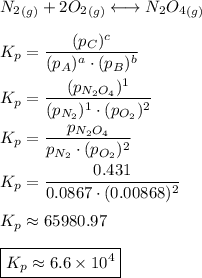Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:20, kristieroth1
Compared with the freezing-point depression of a 0.01 m c6h12o6 solution, the freezing-point depression of a 0.01 m nacl solution is
Answers: 1

Chemistry, 22.06.2019 07:10, angellong94
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

Chemistry, 22.06.2019 10:00, berniceallonce22
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
You know the right answer?
Consider the equilibrium, n2(g) + 2o2(g) < > n2o4(g). calculate the equilibrium constant, kpi...
Questions in other subjects:



English, 31.01.2020 04:52

Mathematics, 31.01.2020 04:52