Chemistry, 08.07.2019 08:00 supergraciepie
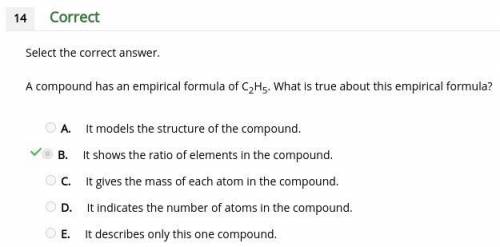
Acompound has an empirical formula of c2h5. what is true about this empirical formula? a. it models the structure of the compound. b. it shows the ratio of elements in the compound. c. it gives the mass of each atom in the compound. d. it indicates the number of atoms in the compound. e. it describes only this one compound.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:30, bbyniah123
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3


You know the right answer?
Acompound has an empirical formula of c2h5. what is true about this empirical formula? a. it models...
Questions in other subjects:

Mathematics, 04.10.2021 02:50


Mathematics, 04.10.2021 02:50

English, 04.10.2021 02:50

Chemistry, 04.10.2021 02:50

Business, 04.10.2021 02:50

Health, 04.10.2021 02:50

Chemistry, 04.10.2021 02:50

Mathematics, 04.10.2021 02:50