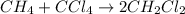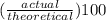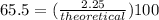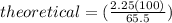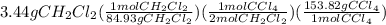Chemistry, 08.07.2019 18:00 bionicboy03120440
It is desired to produce 2.25 grams of dichloromethane (ch2cl2) by the following reaction. if the percent yield of dichloromethane (ch2cl2) is 65.5 %, how many grams of carbon tetrachloride would need to be reacted?carbon tetrachloride methane (ch4)(g) + carbon tetrachloride(g) dichloromethane (ch2cl2)(g)

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:10, ragegamer334p3xlso
Stage in which a star’s outer layers have started to cool and grow outward?
Answers: 3

Chemistry, 22.06.2019 15:00, NatalieKnows
Areaction is first order with respect to reactant x and second order with respect to reactant y. which statement describes the rate law for this reaction?
Answers: 1

Chemistry, 22.06.2019 20:00, denaemarie02
Nitrogen dioxide decomposes according to the reaction 2 no2(g) ⇌ 2 no(g) + o2(g) where kp = 4.48 × 10−13 at a certain temperature. if 0.70 atm of no2 is added to a container and allowed to come to equilibrium, what are the equilibrium partial pressures of no(g) and o2(g)
Answers: 2
You know the right answer?
It is desired to produce 2.25 grams of dichloromethane (ch2cl2) by the following reaction. if the pe...
Questions in other subjects:





Social Studies, 19.05.2021 22:30


History, 19.05.2021 22:30

Mathematics, 19.05.2021 22:30