
Chemistry, 08.07.2019 21:00 khadythiam6957
When solid phosphorus (p4) reacts with oxygen gas, diphosphorus pentoxide is formed. initially 2.87 mol of phosphorus and 3.86 mol of oxygen are combined. (assume 100% yield). a) write a balanced equation for the reaction. b) what is the limiting reactant? c) how many miles of excess reactant remain after the reaction is complete?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, kluckey3426
Asample of air with a volume of 2.20m3 at a pressure of 105 kpa and a temperature of 30c is cooled to 10c and the pressure is reduced to 75.0 kpa. what is the new volume? 6.9 1.34 2.56 43.0 2.88
Answers: 1

Chemistry, 22.06.2019 13:30, nasibamurodova
What are the chemical names of these compounds? ke: mg3n2: reset next
Answers: 1

Chemistry, 23.06.2019 01:30, emfranco1
Which of the following statements is true about energy quantization at the atomic level? electrons in the outermost orbits are the most stable. electrons in all the orbits around the nucleus have the same amount of energy. electrons in the orbit closest to the nucleus have the least amount of energy. electrons absorb or release the same amount of energy independent of the energy levels.
Answers: 1

Chemistry, 23.06.2019 02:00, hermesrobles
Which would freeze at a higher temperature: the great salt lake or lake tahoe? a. lake tahoe would freeze at a higher temperature. b. the great salt lake would freeze at a higher temperature. c. both lakes would freeze at the same temperature.
Answers: 2
You know the right answer?
When solid phosphorus (p4) reacts with oxygen gas, diphosphorus pentoxide is formed. initially 2.87...
Questions in other subjects:




Mathematics, 07.04.2020 20:10

Mathematics, 07.04.2020 20:10

Mathematics, 07.04.2020 20:10



Physics, 07.04.2020 20:10

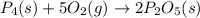
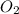 .
.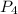 is 0.94 moles.
is 0.94 moles.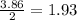 moles of
moles of 

