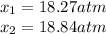Chemistry, 08.07.2019 23:30 juansebas35
for the equilibrium: 2 no (g) < > n2(g) + o2 (g), kp=2400. if initially, only no is present at a partial pressure of 37.30 atm, what will the partial pressures of n2 and o2 be at equilibrium? 1827 atm 38.08 atm 1.725 atm 36.55 atm

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 19:10, asdfghhk9805
How does the atmosphere to make earth livable? check all that apply. causes the seasons contains oxygen provides warmth creates important nutrients blocks harmful energy from the sun plz like !
Answers: 2

Chemistry, 23.06.2019 02:00, jacckiie5176
Which of these is a density dependent factor? a. epidemic b. earthquake c. drought d. hurricane
Answers: 2

Chemistry, 23.06.2019 03:00, kdcloyd3362
Give a real-world example of an energy transformation that uses two of the following forms of energy: chemical, mechanical, nuclear, gravitational, radiant, electrical, thermal (heat), and/or sound.
Answers: 3

Chemistry, 23.06.2019 03:00, yoongislaugh
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3
You know the right answer?
for the equilibrium: 2 no (g) < > n2(g) + o2 (g), kp=2400. if initially, only no is pres...
Questions in other subjects:


Mathematics, 24.01.2021 18:30

English, 24.01.2021 18:40

Mathematics, 24.01.2021 18:40

Health, 24.01.2021 18:40

Mathematics, 24.01.2021 18:40

Mathematics, 24.01.2021 18:40

English, 24.01.2021 18:40


Arts, 24.01.2021 18:40


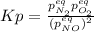
 , changes to:
, changes to: