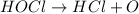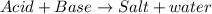Chemistry, 09.07.2019 02:00 mcdowelle360
Apool owner adds a compound containing chlorine to the pool to disinfect it. however, too much of the compound is added. as a result, the water becomes too acidic. what could the pool owner do to reduce the acidity of the pool? he could add another acid to the pool to compete with the existing acid. he could add a metal salt to the pool to precipitate the acid. he could add a base to the pool to neutralize the acid.

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 01:30, alfarodougoy8lvt
Agas is contained in a thick walled balloon when the pressure changes from 1.21 atm to 2.52 the volume changes from 3.75 l to 1.72 l and the temperature change from 293k to blank k
Answers: 3

Chemistry, 22.06.2019 03:40, kellypechacekoyc1b3
Chemical kinetics what was the rate of reaction in trial 3? choose the closest answer.
Answers: 3
You know the right answer?
Apool owner adds a compound containing chlorine to the pool to disinfect it. however, too much of th...
Questions in other subjects:



Mathematics, 28.06.2019 19:30



Biology, 28.06.2019 19:30




Mathematics, 28.06.2019 19:30