

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, estherdinhllama
Which of these best describes the scientific process
Answers: 3

Chemistry, 22.06.2019 19:30, youngdelvin123
Acetylene gas c2h2 undergoes combustion to produce carbon dioxide and water vapor how many grams of water are produced by the same amount of c2h2?
Answers: 2

Chemistry, 22.06.2019 22:30, kristen17diaz
How many valence electrons are in atom of radon?
Answers: 1
You know the right answer?
Complete combustion of 3.60 g of a hydrocarbon produced 11.1 g of co2 and 5.11 g of h2o. what is the...
Questions in other subjects:


History, 13.10.2019 08:00

Social Studies, 13.10.2019 08:00

Mathematics, 13.10.2019 08:00

Biology, 13.10.2019 08:00



Computers and Technology, 13.10.2019 08:00

Mathematics, 13.10.2019 08:00

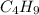 .
.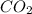 = 11.1 g
= 11.1 g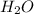 = 5.11 g
= 5.11 g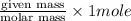 =
= 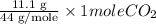 = 0.2522 moles
= 0.2522 moles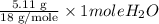 = 0.2838 moles
= 0.2838 moles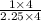 = 4 : 9
= 4 : 9

