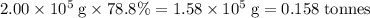Bornite (cu3fes3) is a copper ore used in the production of copper. when heated, the following reaction occurs. 2 cu3fes3(s) + 7 o2(g) 6 cu(s) + 2 feo(s) + 6 so2(g) if 3.24 metric tons of bornite is reacted with excess o2 and the process has an 78.8% yield of copper, what mass of copper is produced?

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, coopera1744
Find the mass in grams of 1.37x1020 particles of h3po4
Answers: 2

Chemistry, 22.06.2019 09:50, Amandachavez94
Although respiratory organs vary across different organisms, they all contain respiratory surfaces that have a large surface area and are extremely thin. explain why having an extremely thin respiratory surface with a large surface area is advantageous for the process of gas exchange
Answers: 1

Chemistry, 22.06.2019 19:30, simihehe
Phosphorous can form an ion called phosphide, which has the formula p3−. this ion can form an ion called phosphide, which has the formula p3−. this ion properties very similar to those of pforms when a phosphorus atom loses three protonsis called a cationcontains 18 electrons
Answers: 2
You know the right answer?
Bornite (cu3fes3) is a copper ore used in the production of copper. when heated, the following react...
Questions in other subjects:

Arts, 10.03.2022 09:10




Mathematics, 10.03.2022 09:10


Mathematics, 10.03.2022 09:10

Mathematics, 10.03.2022 09:20

Mathematics, 10.03.2022 09:20

Mathematics, 10.03.2022 09:20

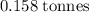
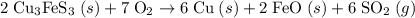
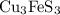 has a molar mass of
has a molar mass of 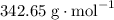 .
. 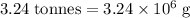 of bornite would thus contain
of bornite would thus contain 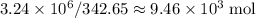 formula units of this substance. Each two formula unit of bornite combusts to produce six formula units of copper metal
formula units of this substance. Each two formula unit of bornite combusts to produce six formula units of copper metal  as seen in the equation;
as seen in the equation; 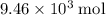 of bornite would therefore produce
of bornite would therefore produce 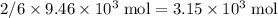 of copper, which corresponds to a theoretical yield of
of copper, which corresponds to a theoretical yield of 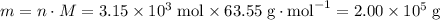 .
. 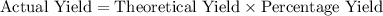 .
.