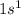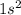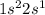Chemistry, 09.07.2019 12:00 loravillanueva87
Which set of these comparisons is incorrect? (more stable means it has a more negative energy.) a. the 1s orbital in h is more stable than the 1s orbital in he+ b. the 2s orbital in he atom is less stable than the 2s orbital in he+ c. the 2s subshell in li is more stable than the 2p subshell in li?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, jonloya264
If 1.8 l of water is added to 2.5l of a 7.0 molarity koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 23.06.2019 10:30, EstherAbuwaah
Identify the limiting reactant when 9.65-g h2so4 reacts with 6.10-g of naoh. the equation is h2s04 + 2naoh = 2h2o + na2so4• what is the theoretical yield of na2so4, in grams? • how much of the excess reagent will remain after the reaction has been completed? • if 10.5-g of na2so4 are actually recovered experimentally, what is the percent yield?
Answers: 3

Chemistry, 23.06.2019 14:00, Gaby702
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 6.00 mol fe and 8.45 mol nio(oh) react?
Answers: 1

Chemistry, 23.06.2019 14:30, hhhhhh8897
If 125 cal of heat is applied to a 60.0-g piece of copper at 25.0 ? c , what will the final temperature be? the specific heat of copper is 0.0920 cal/(g? ? c) .
Answers: 1
You know the right answer?
Which set of these comparisons is incorrect? (more stable means it has a more negative energy.) a....
Questions in other subjects:






Social Studies, 02.07.2019 18:00



Mathematics, 02.07.2019 18:00