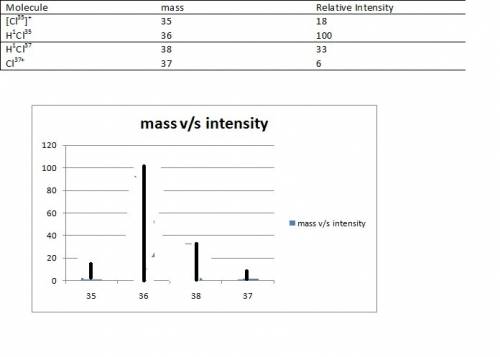
(7 pts) hydrogen and chlorine atoms react to form simple diatomic molecules in a 1: 1 ratio that is hcl. the natural abundances of the chlorine isotopes are 75.77% 35cl and 24.23% 37cl. the natural abundance of 1h is 99.985%, the natural abundance of 2h is 0.015% and there is only a trace (less than 0.001%) of 3h. (a) how many different peaks can hcl produce in a mass spectrometer and what is the mass number for each peak observed? (b) what is the relative abundance of each mass. (c) sketch the mass spectrometry analysis output (relative number of atoms vs mass number) for an hcl sample.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 12:30, AnastasiaJauregui
Which of the following describes a compound? (hint: carbon and oxygen bo a. a piece of pure carbon, containing only carbon atoms b. oxygen gas surrounding a solid piece of carbon c. a substance made of two oxygen atoms for each carbon atom carbon and oxygen atoms mixed without being bonded together
Answers: 1

Chemistry, 22.06.2019 15:40, alleshia2007
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 22:00, cooljariel11
Give more examples of this type of heat transfer:
Answers: 1
You know the right answer?
(7 pts) hydrogen and chlorine atoms react to form simple diatomic molecules in a 1: 1 ratio that is...
Questions in other subjects:

Mathematics, 02.02.2021 21:20



English, 02.02.2021 21:20



Mathematics, 02.02.2021 21:20

Mathematics, 02.02.2021 21:20