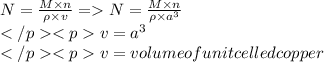To calculate avogadro's number from the measured density of copper shot, what additional information is not needed? the molar mass of copper the atomic number of copper the length of the edge of the unit cell of copper the number of copper atoms occupying one unit cell of copper

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, viktoria1198zz
Complete the following reactions using word and balanced equations including states. dilute phosphoric acid is added with a calcium hydroxide solution.
Answers: 1

Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 16:50, TrueKing184
Answer asap need by wednesday morning explain how a buffer works, using an ethanoic acid/sodium ethanoate system including how the system resists changes in ph upon addition of a small amount of base and upon addition of a small amount of acid respectively. include the following calculations in your i. calculate the ph of a solution made by mixing 25cm3 0.1m ch3cooh and 40cm3 0.1m ch3coo-na+. [ka = 1.74 x 10-5 m] ii. calculate the ph following the addition of a 10cm3 portion of 0.08 m naoh to 500cm3 of this buffer solution. iii. calculate the ph following the addition of a 10cm3 portion of 0.08 m hcl to 200cm3 of the original buffer solution.
Answers: 1
You know the right answer?
To calculate avogadro's number from the measured density of copper shot, what additional information...
Questions in other subjects:


English, 18.07.2019 14:50

Mathematics, 18.07.2019 14:50

Spanish, 18.07.2019 14:50

Computers and Technology, 18.07.2019 14:50


History, 18.07.2019 14:50