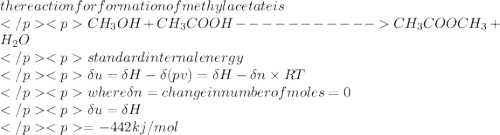Chemistry, 19.09.2019 16:50 clevelandjaniya1
Estimate the standard internal energy of formation of liquid methyl acetate (methyl ethanoate, ch3cooch3) at 298 k from its standard enthalpy of formation, which is –442 kj mol-1.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, jeffcarpenter
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 21.06.2019 18:30, brookekolmetz
How many orbitals does the p sub shell container
Answers: 3


Chemistry, 22.06.2019 04:00, clairebear66
What three natural resources are found in the great lakes region
Answers: 2
You know the right answer?
Estimate the standard internal energy of formation of liquid methyl acetate (methyl ethanoate, ch3co...
Questions in other subjects:

Mathematics, 12.07.2021 21:10

Mathematics, 12.07.2021 21:10

Mathematics, 12.07.2021 21:10

Mathematics, 12.07.2021 21:10

Geography, 12.07.2021 21:10


Biology, 12.07.2021 21:10

Mathematics, 12.07.2021 21:20

History, 12.07.2021 21:20