Chemistry, 09.07.2019 12:30 doggosbepis
The reaction of sulfur with oxygen is shown below. 2s(s) + 3o2(g) → 2so3(g) calculate the mass of sulfur trioxide (in g) produced when 100.0 g of each reactant is present.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:50, iloveballet1857
The electron configuration for chromium is 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 5 4 s 1 instead of 1 s 2 2 s 2 2 p 6 3 s 2 3 p 6 3 d 4 4 s 1 . the configuration is an exception to the
Answers: 3


Chemistry, 21.06.2019 22:30, mimireds5419
1. combine iron and copper (ii) sulfate solution. (hint: iron will form the iron (iii) ion) fe + cuso4 → 2. combine lead (ii) nitrate and potassium iodide solutions. pb(no3)2+ kl → 3. combine magnesium metal and hydrochloric acid solution. mg + hcl → 4. electrolysis (splitting) of water. h2o → 5. burning magnesium. mg + o2 →
Answers: 3

You know the right answer?
The reaction of sulfur with oxygen is shown below. 2s(s) + 3o2(g) → 2so3(g) calculate the mass of su...
Questions in other subjects:


Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01




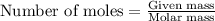 .....(1)
.....(1)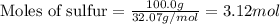
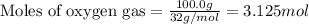
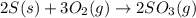
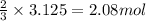 of sulfur metal
of sulfur metal