

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 10:30, shaylawaldo11
Apiece of metal with a length of 1.42 cm was measured using four different instruments. which of the following measurements is the most accurate?
Answers: 3


Chemistry, 23.06.2019 16:30, firstone04kr
Aresearcher wants to experiment with an element that reacts like phosphorus (p) but has a greater atomic mass. which element should the researcher select for the experiment?
Answers: 1

Chemistry, 23.06.2019 18:30, vchery21
Match the following items. match the items in the left column to the items in the right column. 1. 1/1,000 precision 2. uncertainty value of measurement milli- 3. 1,000 accuracy 4. instrument to measure volume balance 5. degree of exactness of a measurement centi- 6. instrument to measure mass graduated cylinder 7. correctness of a measurement ± value 8. 1/100 kilo-
Answers: 1
You know the right answer?
Acompound containing only c, h, and o, was extracted from the bark of the sassafras tree. the combus...
Questions in other subjects:


History, 24.08.2021 18:50


Computers and Technology, 24.08.2021 18:50

English, 24.08.2021 18:50

Mathematics, 24.08.2021 18:50



Mathematics, 24.08.2021 18:50

English, 24.08.2021 18:50

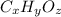 .
.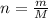
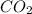 will be:
will be: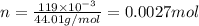
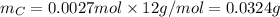
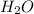 will be:
will be: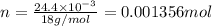
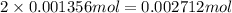 mole of H.
mole of H.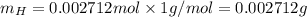
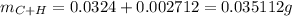
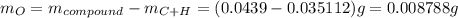
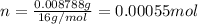
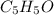 .
.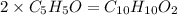 .
.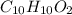 respectively.
respectively.

