Chemistry, 09.07.2019 17:00 oliviastarkweather16
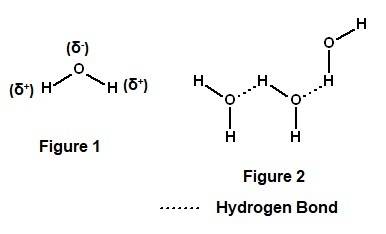
Water molecules have a polarity, which allows them to be electrically attracted to other water molecules and other polar molecules by weak chemical bonds known as view available hint(s) water molecules have a polarity, which allows them to be electrically attracted to other water molecules and other polar molecules by weak chemical bonds known as hydrogen bonds polar covalent bonds van der waals interactions ionic bonds nonpolar covalent bonds

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, angelicar1160
How do scientists think that gravity affected the formation of our solar system?
Answers: 1


Chemistry, 22.06.2019 23:00, brianfranklin17
What is the correct lewis dot structure for arsenic?
Answers: 2

Chemistry, 23.06.2019 00:30, DragonLovely
•hydration •dissociation •dissolving which one goes to which
Answers: 1
You know the right answer?
Water molecules have a polarity, which allows them to be electrically attracted to other water molec...
Questions in other subjects:



Social Studies, 17.07.2019 06:30

Mathematics, 17.07.2019 06:30

Mathematics, 17.07.2019 06:30

Mathematics, 17.07.2019 06:30


English, 17.07.2019 06:30

Physics, 17.07.2019 06:30