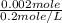Chemistry, 09.07.2019 21:30 brianna4455
Suppose you believe your unknown compound is naoh, and you prepare 20 ml of a 0.1 mol/l solution of it. the stockroom provides a 0.2 mol/l hcl solution as the titrant. what volume of hcl (in ml) is needed to reach the equivalence (stoichiometric) point if your unknown compound is naoh? naoh(aq) + hcl(aq) --> nacl(aq) + h2o(l)

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 05:00, adrian128383
What forms when chemical reactions combine pollution with sunlight?
Answers: 1

Chemistry, 22.06.2019 15:00, hockeykid7583
Which are forms of frozen water? check all that apply. dew frost hail rain sleet
Answers: 2

Chemistry, 22.06.2019 15:10, kolbehoneyman
The ozone molecule o3 has a permanent dipole moment of 1.8×10−30 cm. although the molecule is very slightly bent-which is why it has a dipole moment-it can be modeled as a uniform rod of length 2.5×10−10 m with the dipole moment perpendicular to the axis of the rod. suppose an ozone molecule is in a 8000 n/c uniform electric field. in equilibrium, the dipole moment is aligned with the electric field. but if the molecule is rotated by a small angle and released, it will oscillate back and forth in simple harmonic motion. what is the frequency f of oscillation?
Answers: 2
You know the right answer?
Suppose you believe your unknown compound is naoh, and you prepare 20 ml of a 0.1 mol/l solution of...
Questions in other subjects:



English, 03.08.2019 02:30

Mathematics, 03.08.2019 02:30

English, 03.08.2019 02:30

Social Studies, 03.08.2019 02:30

Geography, 03.08.2019 02:30

Geography, 03.08.2019 02:30


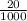 × 0.1
× 0.1