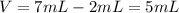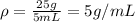Chemistry, 09.07.2019 22:30 jordantay208
An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to 2 ml. the height of the water rose to 7 ml. if the mass of the stone was 25 g, what was its density

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, Unknowndragon42
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 17:40, aaliyahthomas37
Which statement about hf is true? it is zero for any compound in its standard state. it is positive when the bonds of the product store more energy than those of the reactants. it is negative when a compound forms from elements in their standard states. it is zero for any element that is in the liquid state.
Answers: 1

Chemistry, 22.06.2019 23:30, johnnysteeler9934
The ammonia molecule in the diagram has the observed bond orientation because
Answers: 1
You know the right answer?
An irregularly shaped stone was lowered into a graduated cylinder holding a volume of water equal to...
Questions in other subjects:

Mathematics, 11.10.2019 16:00


Mathematics, 11.10.2019 16:00

Mathematics, 11.10.2019 16:00

Biology, 11.10.2019 16:00

Computers and Technology, 11.10.2019 16:00