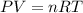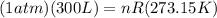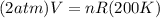Chemistry, 09.07.2019 23:00 erickamurillo9929
10 what is the volume of gas at 2.00 atm and 200.0 k if its original volume was 300. l at stp? 109 .9 l 219.8 l 1.1 l 4.5 l

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:00, rebeccacruzz2017
Calculate - analysis of compound composed of iron and oxygen yields 174.86 of fe and 75.14g of o. what is the empirical formula for this compound?
Answers: 3

Chemistry, 22.06.2019 17:30, kevin72937
Consider the story you just read. all but one of the choices below indicate that something is living.
Answers: 1

Chemistry, 22.06.2019 22:30, pookie879
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3

Chemistry, 22.06.2019 23:30, treylartigue
The appropriate concentration for an iodine sanitizer is
Answers: 1
You know the right answer?
10 what is the volume of gas at 2.00 atm and 200.0 k if its original volume was 300. l at stp? 109...
Questions in other subjects:






English, 05.02.2021 22:20

Mathematics, 05.02.2021 22:20

Mathematics, 05.02.2021 22:20