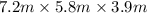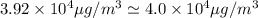Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2

Chemistry, 23.06.2019 12:30, dependentclause5828
Idid a lab for chemistry where we put nails in a copper (ii) chloride solution. 1. why did the reaction stop? which reactant was used up? how do you know? 2. describe what was happening to the atoms of iron and copper during the reaction. what is this type of reaction called? 3. what would happen to the ratio of copper to iron if you had placed more nails in the beaker? if you had let the reaction go for less time? 4. what is the accepted ratio of copper atoms to iron atoms in this reaction? account for differences between your experimental value and the accepted value. write the balanced equation for the reaction.
Answers: 2

Chemistry, 23.06.2019 15:30, Marliii363782
An isotope undergoes radioactive decay. the new isotope that forms has an atomic number fhat is 2 less than the original isotopes. which kind of decay has occured and how do you know
Answers: 2
You know the right answer?
Nail polish remover containing acetone was spilled in a room 7.2 m × 5.8 m × 3.9 m. measurements ind...
Questions in other subjects:


Mathematics, 11.11.2020 07:40


English, 11.11.2020 07:40

Mathematics, 11.11.2020 07:40


Mathematics, 11.11.2020 07:40

Mathematics, 11.11.2020 07:40

History, 11.11.2020 07:40

History, 11.11.2020 07:40