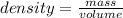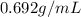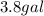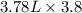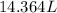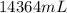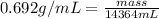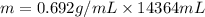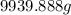Chemistry, 10.07.2019 03:30 messyquestions
Assume a gasoline is isooctane, which has a density of 0.692 g/ml. what is the mass of 3.8 gal of the gasoline (1 gal = 3.78 l)?

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:10, purplefish53
Consider the reaction: n2(g) + o2(g) ⇄ 2no(g) kc = 0.10 at 2000oc starting with initial concentrations of 0.040 mol/l of n2 and 0.040 mol/l of o2, calculate the equilibrium concentration of no in mol/l how would this be done?
Answers: 3

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1


Chemistry, 23.06.2019 06:30, fshane7705
The velocity of any object depends upon a) the location of the object. b) the location of the observer. c) which measurement tools are used. d) the relative motion of the observer.
Answers: 1
You know the right answer?
Assume a gasoline is isooctane, which has a density of 0.692 g/ml. what is the mass of 3.8 gal of th...
Questions in other subjects:

Mathematics, 29.01.2021 02:30

Mathematics, 29.01.2021 02:30

History, 29.01.2021 02:30



Physics, 29.01.2021 02:30


Mathematics, 29.01.2021 02:30