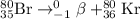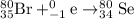Chemistry, 10.07.2019 05:30 22savage2017
He 80br (atomic number 35) nuclide decays either by β− decay or by electron capture. (masses of atoms: 80br =79.918528 amu; 80kr =79.916380 amu; 80se =79.916520 amu. neglect the mass of electrons involved because these are atomic, not nuclear, masses.) (a) write the balanced nuclear equations for each process below. use the isotope tool in the palette to enter both the mass numbers and the atomic numbers for each nuclide.

Answers: 1


Other questions on the subject: Chemistry


You know the right answer?
He 80br (atomic number 35) nuclide decays either by β− decay or by electron capture. (masses of atom...
Questions in other subjects:

Mathematics, 26.03.2021 16:40


Chemistry, 26.03.2021 16:40

Social Studies, 26.03.2021 16:40


Mathematics, 26.03.2021 16:40


Mathematics, 26.03.2021 16:40

Social Studies, 26.03.2021 16:40

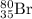 by
by 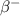 decay is as follows:
decay is as follows: