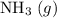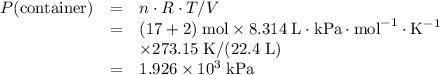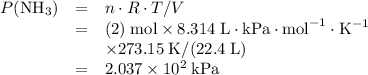Chemistry, 10.07.2019 05:30 sportsseolive4471
Avessel of volume 22.4 dm3 contains 20 mol h2 and 1 mol n2 ad 273.15 k initially. all of the nitrogen reacted with sufficient hydrogen to form nh3. calculate the total pressure and the partial pressure of each component in the final mixture at 273.15 k.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 16:30, joshua1255
Find the number of moles of argon in 364g of argon.
Answers: 2

Chemistry, 22.06.2019 21:00, itasykamila
How many neutrons does an element have if its atomic number is 50 and its mass number is 166
Answers: 1

Chemistry, 23.06.2019 00:30, emilylizbeth12334
Which of the following best describes technology a. something created for only scientists to use b. the method of thinking that scientists use. c. the application of engineering to create useful products. c. a scientific idea
Answers: 1
You know the right answer?
Avessel of volume 22.4 dm3 contains 20 mol h2 and 1 mol n2 ad 273.15 k initially. all of the nitroge...
Questions in other subjects:

Computers and Technology, 10.03.2020 07:01



History, 10.03.2020 07:01






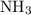 at a
at a 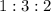 ratio:
ratio: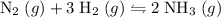
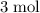 of hydrogen gas would have been consumed while
of hydrogen gas would have been consumed while  of ammonia would have been produced. The final mixture would therefore contain
of ammonia would have been produced. The final mixture would therefore contain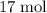 of
of 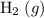 and
and