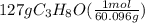Chemistry, 10.07.2019 07:30 sduhaime1974
Isopropanol (c3h8o) is a key ingredient in some hand sanitizers. suppose that 127 grams of isopropanol is dissolved in water. the volume of the solution is 1,250 milliliters. what is the molarity of the solution? refer to the periodic table to you answer. express your answer to three significant figures. the molarity of the solution is m.

Answers: 2


Other questions on the subject: Chemistry

Chemistry, 22.06.2019 07:00, haydjanggg6578
The boiling point of propanoic acid is higher than that of 1-butanol because: propanoic acid has a higher molecular weight than 1-butanol. propanoic acid is more soluble in water than 1-butanol. propanoic acid is a better hydrogen bond donor than 1-butanol. propanoic acid forms hydrogen bonded dimers and 1-butanol does not. 1-butanol forms hydrogen bonded dimers and propanoic acid does not.
Answers: 2

Chemistry, 22.06.2019 10:00, paynedeforest2596
According to the tide table below what time of day will the highest tide occur? (2 pt) the highest tide will occur at
Answers: 1

You know the right answer?
Isopropanol (c3h8o) is a key ingredient in some hand sanitizers. suppose that 127 grams of isopropan...
Questions in other subjects:




Mathematics, 26.04.2021 23:20



Mathematics, 26.04.2021 23:20



 = 3(12.011) + 8(1.008) + 1(15.999) = 60.096 gram per mol
= 3(12.011) + 8(1.008) + 1(15.999) = 60.096 gram per mol