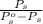An ideal solution is formed from a mixture of the nonvolatile solute urea, co(nh2)2, and methanol, ch3oh. the vapor pressure of pure methanol at 20°c is 89 mmhg. if 6.0 g of urea is mixed with 39.8 g of methanol, calculate the vapor pressure of the methanol solution.

Answers: 1


Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, dwighthibbert56
What was the procedure by which case united states vs lopez went to court
Answers: 1

Chemistry, 22.06.2019 00:30, TMeansStupidity
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 07:30, veronica25681
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1
You know the right answer?
An ideal solution is formed from a mixture of the nonvolatile solute urea, co(nh2)2, and methanol, c...
Questions in other subjects:

Mathematics, 20.05.2021 15:10

English, 20.05.2021 15:10

English, 20.05.2021 15:10

English, 20.05.2021 15:10

Social Studies, 20.05.2021 15:10

Mathematics, 20.05.2021 15:10

Mathematics, 20.05.2021 15:10

English, 20.05.2021 15:10

Arts, 20.05.2021 15:10