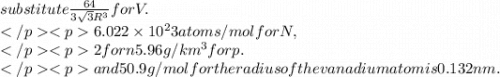Chemistry, 10.07.2019 08:30 dazesreplayy2451
Calculate the radius of a vanadium atom, given that v has a bcc crystal structure, a density of 5.96 g/cm3 , and an atomic weight of 50.9 g/mol

Answers: 1


Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:00, zamariahyou
A5.000 g sample of niso4 h2o decomposed to give 2.755 g of anhydrous niso4. what is the formula of the hydrate? what is the full chemical name for the hydrate? what is the molar mass of the hydrate? niso4•_h2o what is the mass % of water in the hydrate?
Answers: 1

Chemistry, 22.06.2019 15:20, munziruddin204
Which description best characterizes the motion of particles in a solid?
Answers: 2

Chemistry, 22.06.2019 21:30, jpimentel2021
Under which circumstances are kp and kc equal for the reaction aa(g)+bb(g)⇌cc(g)+dd(g)?
Answers: 2
You know the right answer?
Calculate the radius of a vanadium atom, given that v has a bcc crystal structure, a density of 5.96...
Questions in other subjects:




English, 03.11.2019 21:31

Mathematics, 03.11.2019 21:31




Chemistry, 03.11.2019 21:31